Dragonfly Therapeutics to Present Preclinical Data on Clinical-Stage DF6215, its Engineered IL-2R alpha-active Agonist, and DF9001, its EGFR-targ
DF9001 is currently being evaluated for the treatment of Non-Small Cell Lung Cancer, Renal Cell Carcinoma and Head and Neck Squamous Cell Carcinoma
DF6215 is currently being evaluated for the treatment of advanced solid tumors
BOSTON, Nov. 7, 2024 /PRNewswire/ -- Dragonfly Therapeutics, Inc., a clinical-stage biotechnology company developing novel immunotherapies, today announced the company will deliver poster presentations at the Society for Immunotherapy of Cancer (SITC) Annual Conference, highlighting preclinical data supporting two clinical-stage assets, DF6215, its engineered IL-2 cytokine, and DF9001, its EGFR-targeting TriNKET, for the treatment of advanced solid tumors.

Data show that Dragonfly's DF6215 IL-2 cytokine drives greater therapeutic benefit in vivo than non-alpha IL-2, with no evidence of capillary leak syndrome or cytokine release syndrome . In addition, DF6215 synergizes with PD-1 blockade cancer treatment in the "cold" B16F10 melanoma tumor model, without adding toxicity. "DF6215 is a differentiated IL-2, specifically tuned to potently stimulate anti-tumor CD8 T cells and NK cells without incurring counterproductive Treg expansion or VLS," said Ann Cheung, Chief Scientific Officer of Dragonfly Therapeutics.
Dragonfly's DF9001 EGFR-targeting TriNKET drives efficacy via both EGFR-signal inhibition and immune-mediated mechanisms. Data show superior anti-tumor activity via EGFR signal inhibition compared to cetuximab in a xenograft mice model, and that DF9001 induces potent in vivo efficacy via immune effector cells, even in cancer models not dependent on EGFR signaling. DF9001 was well-tolerated at <=50 mg/kg/week in a 4-week GLP study in cynomolgus monkeys. "DF9001 is the only EGFR-targeting agent that allows engagement of both innate and adaptive immune effectors," remarked Cheung, "and does so with a favorable safety profile."
SITC is being held November 6-10, 2024 in Houston, Texas, USA. Dragonfly's poster presentations will take place on Saturday, Nov. 9, Lunch (12:15-1:45 pm), and Poster Reception (7-8:30 pm) in the George R. Brown Convention Center - Level 1 - Exhibit Halls AB.
-- Abstract Number 962: A novel alpha-active IL-2-Fc has expanded therapeutic index and robust monotherapy efficacy in mouse cancer models and strong synergy with PD-1 blockade. The full DF6215 poster is linked here [https://c212.net/c/link/?t=0&l=en&o=4297299-1&h=389869485&u=https%3A%2F %2Fwww.dragonflytx.com%2Fdf6215-poster&a=The+full+DF6215+poster+is+linke d+here] -- Abstract Number 1060: DF9001, an EGFR Targeted Immune Engager, Stimulates Innate and Adaptive Anti-Tumor Immunity with a Distinctive Safety Profile." The full DF9001 poster is linked here [https://c212.net/c/link/?t=0&l=en&o=4297299-1&h=637257415&u=https%3A%2F %2Fwww.dragonflytx.com%2Fdf9001-poster&a=The+full+DF9001+poster+is+linke d+here] About DF6215 DF6215 is a modified, monovalent, half-life extended recombinant human IL-2 that biases toward activation of anti-tumor T and NK cells in order to improve upon the benefit-to-risk ratio of historic IL-2 drugs.
DF6215 was found to:
-- Increase proliferation of immune cells and preferentially expand anti-tumor effector cells compared to non-alpha IL-2 molecules (maximizing the anti-tumor effector:Treg ratio) -- Increase granzyme B expression in tumor-infiltrating CD8+ T cells and NK cells -- Demonstrate effective therapeutic efficacy in mouse cancer models as a single agent as well as in combination with immune checkpoint blockade -- Preferentially expand activated CD8+ T cells in NHP without evidence of capillary leak syndrome or cytokine release syndrome DF6215 is the second in a pipeline of cytokines that Dragonfly is developing to address unmet need in patients with advanced cancer and other diseases. Dragonfly's DF6215 Phase 1/1b clinical trial is a first-in-human, multi-part, open-label study to investigate the safety, tolerability, pharmacokinetics, biological, and clinical activity of DF6215 in patients with advanced (unresectable, recurrent, or metastatic) solid tumors (NCT06108479).
About DF9001 DF9001 is an investigational first-in-class multi-specific drug candidate that targets and inhibits EGFR and potently redirects natural killer (NK) cells, gamma-delta T cells, and CD8 T cells by engaging activating receptors NKG2D and CD16.
DF9001 disrupts tumor growth by inhibiting EGFR signaling and promoting anti-tumor immunity. It has been optimized for engagement of immune cell populations that lead to the direct killing of tumor cells, while sparing healthy tissues, as well as the production of chemokines and cytokines that recruit additional effector cells to kill tumor cells. These characteristics make DF9001 a promising therapeutic agent against EGFR+ cancers, particularly those for which EGFR signal inhibition alone is ineffective.
DF9001 was discovered and developed using Dragonfly's TriNKET® platform. DF9001 is being evaluated in adult patients for the treatment of advanced solid EGFR-positive tumors (NCT05597839). DF9001 has the potential to stimulate anti-tumor immunity in patients who are not eligible or not adequately responding to current therapies. It is the second wholly owned drug candidate in a pipeline of TriNKETs Dragonfly is developing to address high unmet needs for patients across a broad range of disease areas.
About Dragonfly Dragonfly Therapeutics is a clinical-stage biopharmaceutical company committed to discovering, developing and commercializing novel therapies that harness the body's immune system to bring breakthrough treatments to patients. In addition to a set of advanced programs in the clinic, Dragonfly has a deep pipeline of wholly owned preclinical candidates discovered using its proprietary platforms that are progressing toward the clinic, as well as productive collaborations with Merck, AbbVie, Gilead and Bristol Myers Squibb in a broad range of disease areas.
For more information visit www.dragonflytx.com.
Dragonfly Contact Anne Deconinck anne@dragonflytx.com
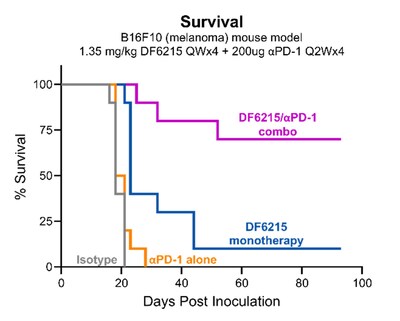



Photo - 
Photo - 
Photo - 
Photo - 
Logo - 
Andere persberichten of expertquotes van deze organisatie:

Dit is een origineel bericht van PR Newswire
Ga naar alle berichten van deze organisatie.
